Sodium-ion Batteries: The Next Revolution in Energy Storage?
Technical Analysis | 02-05-2023 | By Liam Critchley
Sodium-ion (Na-ion) batteries have a lot of promise and join the list of the other metal-ion batteries that have not yet made it to the commercial heights of lithium-ion (Li-ion) batteries. However, as more and more people use lithium, there may come a point where resources become scarce, and other technologies need to be available as alternatives. There’s a lot of potential for sodium-based batteries due to the low cost of sodium, the abundance of sodium deposits, and the ability to create batteries with a high energy density if some of the current issues are ironed out.
The Potential of Sodium-ion Batteries
Despite the potential for the high energy storage density of Na-ion batteries, there are still a number of issues surrounding rate performance, Coulombic efficiency (a measure of how effectively a battery can convert electrical energy during charging and discharging), and cycle stability that have hampered their commercialisation. The main reason is that sodium has a larger atomic radius than lithium, which results in slower reaction kinetics during the sodiation (the process of sodium ions entering the anode) and desodiation (the process of sodium ions leaving the anode) mechanisms between the sodium ions and the anode This means that Na-ion batteries will require anodes with microscopic-scale internal structures to house the sodium ions during the charge and discharge processes while remaining compatible with the current electrolytes used today. One option is to use manganese oxide anodes, but this has presented its own set of challenges, so researchers have now taken to creating magnesium oxide-graphene aerogel hybrid anode materials to try and solve these challenges.
Manganese Oxide: A Potential Anode Choice
Transition metal oxides, which generally offer high energy density and are typically abundant, have been frequently suggested as potential Na-ion anodes. One of the main anode materials of interest is manganese oxide because of its high theoretical capacity and low cost, but like many other transition metal anodes, their low electrical conductivity and tendency to severely expand in volume during the charge/discharge process have so far led to a poor electrochemical performance.
The real-world implications of overcoming these challenges are significant. The development of efficient sodium-ion batteries could lead to more affordable and sustainable energy storage solutions, impacting various industries such as electric vehicles, renewable energy, and consumer electronics. As the demand for energy storage continues to grow, the successful commercialisation of Na-ion batteries would play a crucial role in meeting global energy needs while reducing reliance on lithium-ion batteries and their associated environmental concerns.
While different anode strategies have so far been unsuccessful, the high theoretical specificity (1018 mAh g-1) of manganese oxide shows promise, but challenges remain in overcoming the poor cycling stability and Coulombic efficiency during charging/discharging due to the disintegration of the anode over time. A lot of effort has been made to overcome these challenges, and nanoparticles have been utilised inside manganese oxide anodes to provide a large electrode–electrolyte contact area and a shorter ion and electron transport distance. Nanocomposites have also been utilised to improve the overall electrical conductivity of these anodes, where the nanofiller material acts as a buffer for accommodating volume changes within the anode. Following on from the success of different nanomaterials in these anodes, researchers have now turned to graphene-based aerogels.
Integrating Graphene Aerogels into Manganese Oxide Anodes
Graphene oxide aerogels—gel-like materials where the liquid within the ‘gel’ has been replaced with air—have gained a lot of interest in different applications due to their high surface area, electrical properties and lightweight nature. The researchers have turned to reduced graphene oxide (rGO) aerogels to try and improve the electrochemical performance of Na-ion batteries.
The rGO aerogel was chosen as a composite anode material because it offers a way of shortening the transportation path for sodium ions via its porous structure—and the pore sizes can be easily tailored to create different sizes, including microporous, mesoporous, and microporous-sized pores. The porous network also offers a way of combatting against any volume changes because the volume expansion will move into the air pockets, preventing the degradation and disintegration of solid anode materials.
There is one problem with using an oxygenated version of graphene, and it is that the electrochemical properties are not as efficient as pristine graphene. However, this can be easily fixed through doping with heteroatoms (atoms other than carbon or hydrogen) such as nitrogen and sulphur to improve the specific capacity (the amount of charge a battery can store per unit of mass) and electronic conductivity of these aerogels.
Integrating Graphene Aerogels for Enhanced Performance
The researchers created a hybrid anode composed of manganese oxide (Mn2O3 structure) with layers of graphene oxide being doped with sulphur atoms and nitrogen atoms. The doped rGO layers were created by heating graphite oxide in the presence of ammonia, L-Cysteine, and manganese oxide particles. The product is self-assembled into highly stable 3D networks featuring hydrogen bonds, coordination, and electrostatic interactions. The manganese oxide particles were distributed on either side of the aerogel layers.
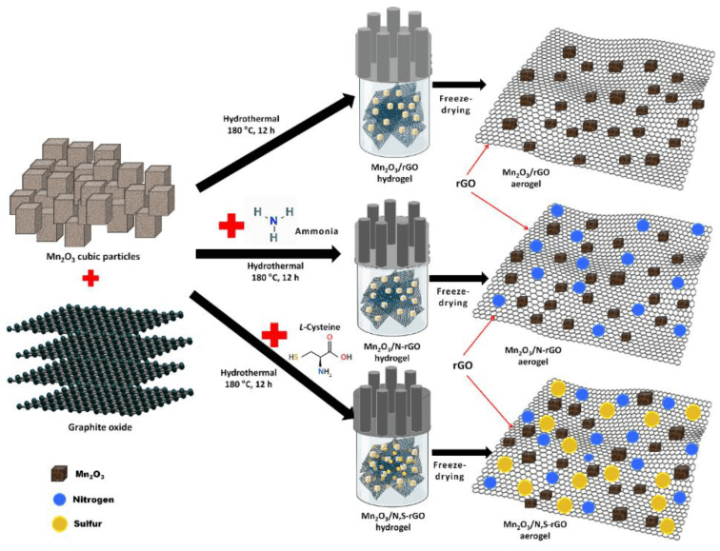
Scheme 1 illustrates the process of synthesising Mn2O3/rGO aerogels and Mn2O3/heteroatom-doped rGO aerogels through a schematic representation.
The porous nature of the manganese oxide/rGO aerogel was utilised and provided assistance to the sodium ions when they migrated in and out of the cathode, while the doped rGO layers offered a conductive network that was advantageous for electron transportation once the ions had been absorbed into the anode.
Device Performance and Future Prospects
In terms of the device performance, the device exhibited a discharge capacity of up to 100 cycles with a Coulombic efficiency of up to 99% compared to undoped rGO. A number of doped-sheet aerogel anode combinations were trialled, and after 100 cycles, the electrodes retained capacities of 242 mAh g-1 for Mn2O3 /rGO aerogels, 325 mAh g-1 for Mn2O3/nitrogen-rGO aerogels, and 277 mAh g-1 for Mn2O3/nitrogen, sulphur-rGO aerogels.
The integration of heteroatom-doped rGO to the manganese oxide materials increased the electrical conductivity of the electrode and the speed of ion transport in the electrode while acting as a buffer for any induced volume changes during operation. The result was a high capacity and stable cycling performance that has been attributed to the synergistic effects between the heteroatom doping and 3D porous network, as these two aspects brought a lot of different benefits to the anode.
While the devices are not yet ready for commercialisation in terms of performance and scalability, the research demonstrates the impact of nanocomposites and nanomaterials on the performance and stability of Na-ion batteries, addressing two of the main challenges in the field." While it may be some time before they are anywhere near the levels of commercial batteries, it may be that finding new nanomaterial-additive routes could be the key to eventually making Na-ion batteries a lot more stable and better performing so that they might one day be used commercially alongside the conventional battery technologies seen today.
In the meantime, the progress made in this research could inspire further investigations into other potential applications of heteroatom-doped reduced graphene oxide aerogels and similar nanomaterials. For instance, their unique properties might be harnessed for use in supercapacitors, fuel cells, or even environmental remediation technologies. As science writer Liam Critchley often emphasises, the cross-disciplinary nature of chemistry and nanotechnology opens up a myriad of possibilities with the potential to revolutionise industries and improve our daily lives.
Reference:

