Detecting Forever Chemicals: New Luminescent Sensor Insights
Insights | 12-02-2024 | By Robin Mitchell
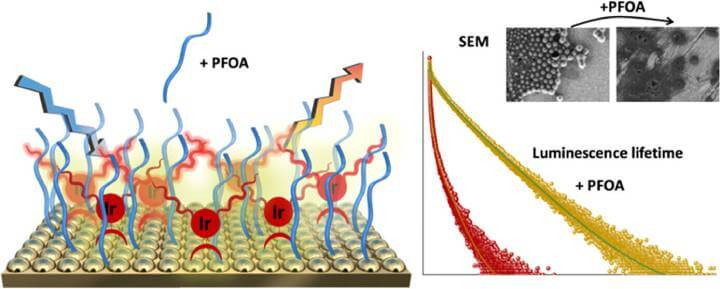
Luminescence lifetimes, known for their sensitivity and stability, make an effective method for detecting chemicals like PFOA, a concerning 'forever chemical' due to its toxicity. We introduce a novel luminescence sensor crafted from iridium-coated gold surfaces, enhanced with IrC6 and IrC12 iridium complexes and Zonyl-FSA surfactant, for precise PFOA detection. This sensor, responsive to environmental changes, significantly alters its red luminescence signal upon PFOA contact, enabling accurate monitoring of its concentrations in water. Tested in various samples, it successfully identifies PFOA levels above 100 μg/L, offering a rapid, accurate, and cost-efficient solution for environmental monitoring. Credit: Analytical Chemistry (2024). DOI: 10.1021/acs.analchem.3c04289
Key things to know:
- Innovative luminescent sensor technology has been developed to detect 'forever chemicals' like PFOA in water, offering a rapid and effective solution for identifying pollution sources.
- This new sensor utilizes iridium and gold to create a luminescent reaction when exposed to UV light and PFOA, changing the light emitted and indicating the presence of these harmful chemicals.
- The technology aims to significantly improve environmental protection by providing a cost-effective, simple, and quick method for monitoring water quality and safety.
- Collaboration between UK and German researchers highlights the global effort to combat pollution and protect public health from the adverse effects of industrial contaminants.
A leap forward in detecting forever chemicals
Recognising the challenges faced by forever chemicals in the environment, researchers have recently demonstrated a new luminescent sensor that aims to allow for the rapid detection of these chemicals, thereby allowing for sources of pollution to be found faster. What challenges do such chemicals present, what did the researchers develop, and how could such technologies transform environmental protection?
What challenges do forever chemicals present?
Ever since man first shaped stones to create tools, our ability to extract resources from the natural environment has been second to none. While every other life form on earth exists in its natural habitat in a degree of harmony, humans have an incredible talent for not only making the most of their environment but also shaping it to their will.
An excellent example of this was how early England used to be covered in dense forests as far as the eye could see. While this may have been advantageous for wood and other resources, it also made hunting especially difficult, with bushes and dense trees making it hard to spot game.
To combat this, massive amounts of land were turned into grasslands and meadows while forests became managed, with only so many trees being allowed to grow. By doing so, hunters could easily spot game in the fields and through trees.
This ability to manipulate the environment doesn’t just stop there; the drive for discovery and invention has allowed man to find all kinds of ways of extracting every possible resource from the environment, whether it is on the land, in the sky, or deep below. But for all the advantages that this ability has provided, it has come at a great cost to the environment as well as human health.
The Environmental Cost of Human Progress
One such case of this damage that continues to introduce serious issues is the use of “forever chemicals” and how they easily leak from industrial processes, products, and waste into the environment. The term “forever chemicals” implies that, unlike many chemicals that eventually break down into safe components, forever chemicals can remain in the environment indefinitely. Furthermore, this term is often applied to chemicals and compounds that can cause harm to human health, whether it’s through the formation of cancer, damaging organs, or causing harm to development.
The persistence of 'forever chemicals', particularly PFAS (Per- and polyfluoroalkyl substances), in the environment poses a significant challenge due to their non-degradable nature. These chemicals, used widely across various industries for their oil and water repellency, accumulate in the environment, leading to concerns over toxic pollution, especially in water sources. The recent development of a luminescent sensor by researchers aims to address the urgent need for a simple, rapid, and cost-effective method to detect these pollutants, highlighting the importance of innovative technologies in combating environmental contamination.
While environmental standards surrounding what chemicals can be used in products and their eventual disposal have significantly improved over the years, the fact that forever chemicals remain in the environment indefinitely makes them problematic for decades. Furthermore, as such chemicals can cause issues at extremely low concentrations (parts per million), it can be hard to identify these compounds rapidly, requiring expensive testing.
Researchers develop luminescent sensor for finding forever chemicals
Recognising the dangers faced by forever chemicals and the challenges in detecting them, researchers from the UK and Germany have teamed up to develop a new luminescent sensing device that is able to detect these chemicals at low concentrations.
The new device, which is currently in its prototyping stages, is able to detect minute amounts of a specific chemical called perfluorooctanoic acid (PFOA), which is commonly found throughout numerous industries and is believed to be a potential carcinogen. The device consists of luminescent metal complexes that are attached to the sensor surface, utilising iridium and gold. When exposed to UV light, the iridium emits red light that is then detected with a photoreceptor.
To better understand the innovative mechanism behind the detection of PFOA, let's examine a detailed illustration of the technology in action. The following figure provides a visual representation of how the sensor's functionalized gold surfaces interact with PFOA, showcasing the critical process of micelle formation and disassembly. This visual aid elucidates the sensor's ability to detect the presence of 'forever chemicals' with precision.
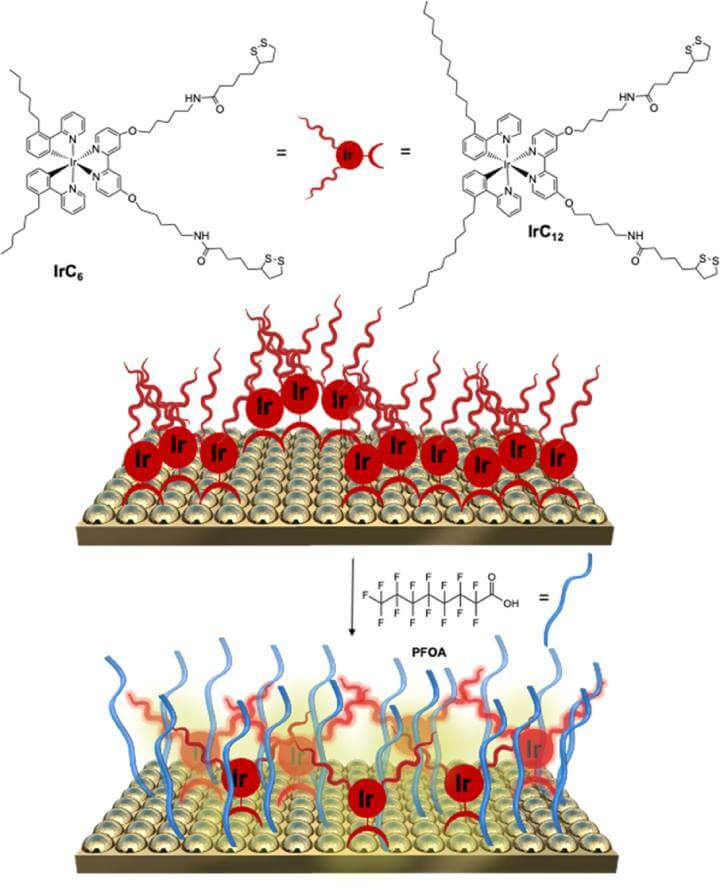
Figure 1: Illustration of gold surfaces functionalised with IrC6@Au and IrC12@Au reacting to PFOA. The diagram shows micelle formation through aggregated complexes at the top, and at the bottom, it demonstrates the primary molecular coverage when exposed to the analyte, emphasising the micelles' breakdown.
When submerged in water containing PFOA, the resulting emission of red light is altered, which in turn adjusts the output from the sensor. According to the researchers, their prototype device is able to detect concentrations of PFOA down to 220µg per litre (approximately 0.2 ppm).
The innovative approach of using luminescent metal complexes, specifically iridium modified on gold surfaces, represents a significant advancement in the detection of PFOA in water. This method's sensitivity to changes in the luminescence signal upon exposure to PFOA allows for the monitoring of pollution levels in real-time. Such advancements are crucial for environmental protection efforts, offering a new tool for rapidly identifying and mitigating the impact of 'forever chemicals' in our ecosystems.
However, the researchers noted that while the ability to detect PFOA in real-time is a significant step in fighting sources of pollution, it would need to be able to detect these chemicals in the nanograms per litre if being used with drinking water.
How could such technologies transform environmental protection?
Protecting the environment from pollutants is unquestionably important, as we need to ensure not only the longevity of our own lives but also the lives of our children and the planet that they will inherit. While the introduction of legislation and regular testing of soils and waters can help find sources of pollutants, the inability to get this data in real time holds back our ability to make critical changes.
If the sensor developed by the researchers can be combined with IoT technologies, it becomes possible to deploy such sensors en-masse, allowing for researchers to generate real-time views of pollutants, where they are coming from, and how they move throughout water sources. Furthermore, such sensors would also allow authorities to respond to chemical dumps and spills as they happen, which not only allows for such incidents to be addressed immediately but also identify those who are responsible.
Overall, what the researchers have developed still requires further refinement, but if the technology can be expanded to other pollutants, it could very well help accelerate methods of environmental protection and how we deploy technology to help with this.
The collaboration between scientists from the University of Birmingham and the Bundesanstalt für Materialforschung und -prüfung (BAM) in Germany underscores the global effort required to tackle the issue of 'forever chemicals'. By focusing on the development of portable and sensitive detection technologies, such as the luminescent sensor for PFOA, researchers are paving the way for more effective environmental monitoring and protection strategies. This cross-disciplinary effort not only enhances our ability to respond to chemical spills and pollution but also contributes to the broader goal of safeguarding public health and the environment from the adverse effects of industrial pollutants.

