Predicting Sepsis with Machine Learning and Lab-on-a-Chip
Technical Analysis | 31-07-2025 | By Liam Critchley

Conceptual illustration: AI-enhanced microfluidic diagnostic device for rapid sepsis detection (AI-generated image, not a real-world photograph of the actual PREDICT platform).
Key Things to Know:
- Sepsis remains a global health emergency, with over 48 million cases annually and a high rate of misdiagnosis in early stages.
- A new microfluidics-based diagnostic platform uses a six-gene RNA signature and machine learning to predict deterioration within 3 hours of patient presentation.
- The PREDICT system achieves 88% accuracy using just a 50 µL blood sample, without the need for lab-based processing or trained specialists.
- This point-of-care tool enables earlier intervention by identifying at-risk patients before traditional symptoms escalate, supporting faster clinical decisions.
Sepsis (blood poisoning) is a life-threatening condition that occurs when the body has an abnormal response to an infection, leading to the body damaging its own tissues and organs. Sepsis is a complex condition that is caused by a dysregulated response to an infection. Estimates since 2017 have shown that there are around 48.9 million cases of sepsis around the world each year, with 11 million of those cases resulting in death (not including deaths from COVID-sepsis).
Early identification, intervention and treatment can significantly reduce mortality rates, as well as prevent the chance of long-term aftereffects (including long-term disability) if the patient does survive. Unfortunately, it's a condition that is often missed early on. Researchers have now developed a machine learning-enabled microfluidics platform that aims to provide point-of-care (POC) services for identifying hospital patients who are at risk of developing sepsis as their condition deteriorates.
Early Diagnosis is Key: But it is a Challenge
Early diagnosis is critical for a high survival rate. There are many methods used today to diagnose sepsis, from PCR methods (made famous during COVID) to clinical algorithms that are based on different tests and risk factors in a patient's medical record, and testing for different biomarkers in the patient's blood―including the shape of white blood cells. However, many tests are not great at identifying if a patient is at risk of developing sepsis while in hospital and can only detect if a patient has developed sepsis and is at a more advanced stage.
Many illnesses can be tested at a patient's bedside (such as glucose monitoring for diabetes). However, for sepsis, the majority of tests require samples to be sent off to specialised laboratories that require specialist equipment and trained operators. This makes the tests less accessible for hard-to-access populations and people who are in regions without these facilities. It's been estimated that sending the samples for testing can delay the diagnosis of sepsis by more than 6 hours, meaning that treatment only gets to patients when it has become more advanced.
Microfluidics and Machine Learning Combine for Quicker Sepsis Diagnosis
To overcome the need to send off samples for testing, lab-on-a-chip (LOC) technology has been developed for a number of ailments and clinical conditions, opening up testing capabilities to a lot more people who don't have access to specialist care centres. LOC systems are small and compact and can be used for POC testing―at a patient's bedside or even in their home―and enable non-trained professionals to administer the tests.
Recent developments have shown that LOC platforms integrated with machine learning can execute complex bioanalytical tasks typically confined to central laboratories. By leveraging small-volume whole blood samples, these systems are capable of near-patient RNA biomarker detection—an approach that aligns with the World Health Organization's goal to decentralise diagnostics and improve care for vulnerable populations.
Enabling Early Risk Stratification with Sepset
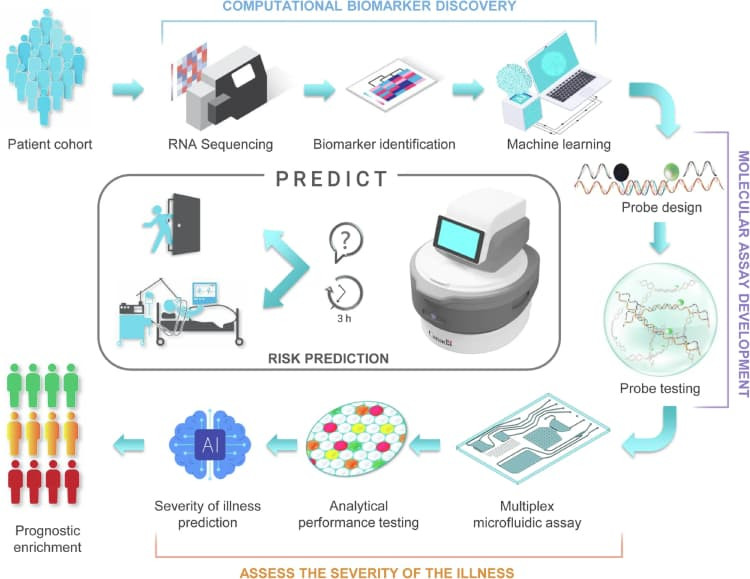
The researchers discovered that a reduced gene signature can distinguish patients who are suspected to have sepsis and are at a high risk of clinical deterioration. Identifying patients at this stage before severe deterioration occurs could help to save lives. They had already developed a centrifugal-based LOC system in a previous study that can automatically extract and identify biomarkers in a blood sample. This system was combined with machine learning algorithms and RNA-based biomarkers to predict which patients were most at risk. The biomarker signatures were classified into two different deterioration risk groups to improve prediction accuracy.
The six-gene classifier, termed Sepset, was selected through rigorous feature reduction from a 99-gene signature associated with immune reprogramming. This reduction was achieved via machine learning techniques, including eXtreme Gradient Boosting (XGBoost), optimised for high sensitivity and balanced accuracy across varied clinical datasets. The classifier was found to be effective not only in standard ICU cases but also among ER patients, offering an early window into disease progression risk.
The device developed in this study, known as the PREcision meDIcine for CriTical care (PREDICT) device, could determine which patients would likely go on to develop sepsis within the next 3 hours using only a 50 µL blood sample.
How the PREDICT Platform Works
The PREDICT platform operates on a centrifugal microfluidics system that automates RNA extraction, digital droplet PCR amplification, and fluorescence-based detection without requiring manual sample preparation. This self-contained system supports both sample-to-answer workflows and high-throughput triaging, using embedded imaging and proprietary thresholding algorithms for real-time decision support.
Testing began with in-house RNA sequencing data, narrowing the target biomarker down to a six-gene expression signature of immune cell reprogramming. Machine learning algorithms were trained on 873 patient transcriptomes and tested against an additional 1241 samples. A digital drop PCR assay was then developed to detect the relevant genes, and a microfluidic cartridge-based platform was created to deliver a positive or negative test result. The raw analysis data was processed via machine learning to estimate the likelihood of clinical deterioration within 24 hours.
Accuracy and Real-World Validation
Importantly, the test does not rely on traditional markers such as lactate, which have shown limited discriminatory power in early sepsis prediction. Instead, the classifier is informed by immune cell transcriptomic profiles that reflect underlying dysfunction rather than surface-level severity indicators, allowing it to flag patients who may otherwise present as clinically stable.
The researchers trialled the platform with blood samples from 586 suspected sepsis patients. The fully automated PREDICT device achieved a sensitivity of 92%, specificity of 89%, and overall accuracy of 88% in predicting imminent risk of deterioration.
To provide a clearer picture of how the classifier was developed and validated, the figure below outlines the complete process—from identifying relevant biomarkers and feature reduction, to real-world application of the PREDICT platform for bedside risk prediction.
In comparative assessments, Sepset demonstrated higher sensitivity than other molecular diagnostic tools such as Septicyte and IMX-SEV, particularly in time-critical scenarios. This predictive robustness enables earlier escalation of care and supports clinical decision-making in resource-constrained settings.
Towards Faster, Easier Sepsis Detection
The PREDICT test represents a step forward in making sepsis diagnosis faster, easier, and more accessible. Unlike traditional tests that require samples to be transported to specialised labs—often delaying diagnosis by hours—the LOC device provides results directly at the patient’s bedside. Because the analysis is fully automated and powered by trained machine learning algorithms, it can be conducted by any clinical professional without additional specialist input.
This approach has the potential to significantly reduce diagnostic delays, enable quicker treatments, and ultimately save lives. As further developments refine the technology, LOC devices like PREDICT could become a standard tool in hospitals and remote care settings for early identification of sepsis risk.
Reference:

