MIT's Ingestible Pill: Advancing Sleep & Depression Diagnosis
Insights | 04-01-2024 | By Robin Mitchell
Measuring bodily functions can be challenging at the best of times, especially during sleep studies for patients trying to diagnose sleep apnoea. Recently, researchers have demonstrated a new pill that has the ability to measure bodily functions without the need for invasive equipment. What challenges do tests involving sleep face, what did the researchers develop, and could such technologies be the way forward?
What challenges do tests involving sleep face?
When it comes to collecting medical data from patients, doctors have an unbelievably wide range of options available. Thermometers can be used to determine body temperature, blood samples can be used to identify the contents of blood, MRIs can be used to generate images of internal structures, and X-rays can be used to find the tiniest fractures in bone.
Taking these tests when patients are awake is fairly straightforward (except for blood tests where those frightened of needles may cause some difficulty), but trying to record data from sleeping patients can be problematic, if not extremely challenging. For example, attaching a series of electrodes to a sleeping patient can cause sensor errors during movement, and considering that movement is extremely common during sleep, it can make getting quality data difficult.
Another challenge faced with taking sensor readings during sleep is the general lack of comfort faced by patients. In the case of sleep apnoea tests, patients are required to wear multiple devices along with bands around their chest to record breathing and a tube placed in or near the nose to measure airflow. While it is not impossible to sleep attached to this equipment, it can certainly make it difficult for patients and, in some cases, can result in agitation and distress.
Even in normal hospital environments, electrodes attached to patients to measure heart rate can be incredibly inconvenient, resulting in a significant restriction of movement. Simple tasks such as going to the bathroom can require medical assistance. Otherwise, patients who disconnect their sensors could trigger false alarms, with crash teams barging into the room thinking that a patient has flatlined.
Finally, continuing advances in technology have allowed patients to take sleep tests from home, utilising internet technologies to transmit recorded data to hospitals. However, while this move does help reduce the burden on hospital staff, it also means that patients need to use testing equipment themselves, which can be difficult. As such, patients who incorrectly use such equipment may send false data to doctors, thus resulting in incorrect diagnoses.
Researchers create ingestible pill for internal body measurements
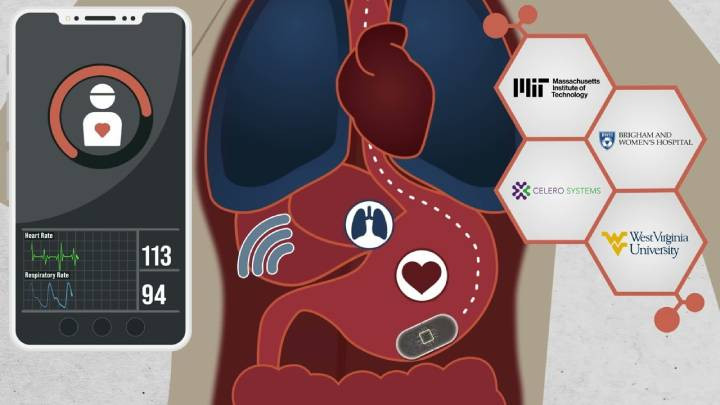
Recognising the challenges faced with sleep tests and medical tests in general, researchers from MIT, Celero Systems, and West Virginia University have recently created a developmental pill that is not only able to be swallowed with ease but also records bodily functions suitable for use in the diagnosis of conditions including sleep apnoea.
The pill-shaped device (which itself is no bigger than a regular vitamin) utilises an accelerometer to measure both heart rate and breathing. This works thanks to the high g-force exerted during beats and the low-frequency forces during breathing, meaning that a single accelerometer can be used to identify both readings simultaneously.
The device was tested amongst multiple humans and showed that it could accurately detect sleep apnoea in patients. However, the researchers responsible for the device also mentioned that it could be used for detecting opioid overdoses in the future, likely via rapid fluctuations in heart rate and sudden changes in breathing.
Advanced Monitoring Capabilities of the Ingestible Device
The ingestible capsule, developed by a collaborative team from MIT, Celero Systems, and West Virginia University, represents a significant leap in medical monitoring technology. This multivitamin-sized device utilises an accelerometer to accurately measure both the breathing rate and heart rate from within the patient's GI tract. Its potential applications extend beyond sleep apnoea diagnosis to include the monitoring of high-risk opioid overdose patients.
Associate Professor Giovanni Traverso from MIT, also a gastroenterologist at Brigham and Women’s Hospital, highlights the device's potential in providing early detection of respiratory changes due to various conditions such as asthma or COPD. In a clinical trial involving ten human volunteers, the capsule demonstrated its ability to monitor vital signs effectively and detect sleep apnoea episodes without causing any adverse effects to the patients.
Furthermore, the research team, including renowned figures like Robert Langer of MIT's Koch Institute for Integrative Cancer Research, envisions this sensor as a less intrusive method for diagnosing sleep apnoea. It could also play a crucial role in monitoring treatment effectiveness for apnoea patients. The device's development by Celero Systems is a testament to the evolving landscape of medical technology, where patient comfort and accurate monitoring coalesce.
While the device was shown to be a success, the fact that it is ingested means that it can only spend a day or two inside the body before being passed on. But even though this may seem like a drawback of the technology, it also means that nothing is left in the body for too long, making it ideal for use where immediate data is required over a short period of time.
Finally, the researchers also noted that it may be possible for future versions of the pill to dispense medication as needed. Going back to the example of opioid overdoses, the pill could detect such an overdose and secrete naloxone to restore proper breathing.
Could such technologies be the way forward in medicine?
Ingestible sensors and medical systems are by no means new; researchers have been working on such technologies for decades and with great success. However, what the new device does differently is that it is able to infer numerous biomarkers from a single sensor, all while being entirely non-invasive.
If the technology demonstrated by the researchers can be proven in more trials while made cost-effective, it could very well be the next form of testing in patients. Of course, it will be importable that such devices are manufactured cheaply as such devices would be a one-time use device, and it is highly unlikely that hospitals will be looking for excreted devices in their sewer system for the purpose of recycling.
But that does lead to a series of important questions: if such devices could be mass-produced, would our sewage system be full of monitoring devices, and what implications would that have? How could such devices be made biodegradable without harming the human body? Finally, would consumers want these devices recycled, and if not, how could they be safely disposed of?
Future Developments and Ethical Considerations
Looking ahead, the researchers aim to integrate an overdose reversal agent, such as nalmefene, into the capsule. This advancement could enable automatic drug release when a patient's breathing rate slows or stops, offering a groundbreaking solution in emergency scenarios. Additionally, efforts are underway to prolong the capsule's retention time in the stomach, enhancing its monitoring capabilities.
The ethical implications of such advanced technology are significant. While offering unparalleled monitoring and treatment options, questions arise about patient privacy and the long-term impact of such devices on healthcare practices. As we embrace these innovative solutions, it is imperative to balance technological progress with ethical responsibility, ensuring that patient welfare remains at the forefront of medical advancements.

