Super hydrophobic cooling offers greater performance for high power electronics
| 21-05-2018 | By Rob Coppinger
High-power electronics could work at higher temperatures for longer with water flowing over a heat exchanger’s surface that is designed to repel the liquid, the exact opposite of what is used today.
Water is typically boiled off hydrophilic heat exchanger surfaces by the heat of the high-power electronics, keeping them cool. A hydrophilic surface attracts water. A hydrophobic surface repels water, but it could be a better approach to cooling for the high-power electronics used in electric cars, supercomputers and in future all-electric aircraft.
“Using a superhydrophobic surface, the thought is it would be ten times worse [than hydrophilic] and what we showed was that with the appropriate treatment of the surface, you can make it significantly better,” Justin Weibel, an associate research professor of mechanical engineering at Purdue University, told Electropages.com.
The Purdue University research found that superhydrophobic materials can boil water efficiently under the right conditions and stay cooler than hydrophilic surfaces. The heat on a surface, its energy density, is measured by Watts per centimetre squared and referred to as the heat flux. High-power electronics at a normal level of power can, for example, operate with a heat flux of 500W per square centimetre. With hydrophobic cooling it could be possible for electronics to consume twice their normal amount of power but have no change in their surface temperature.
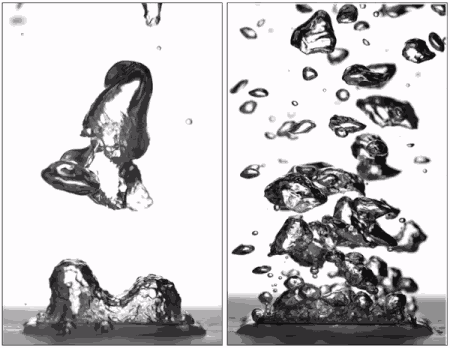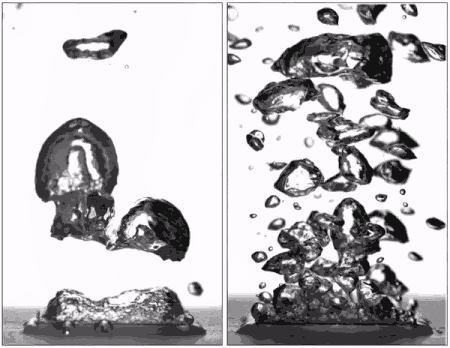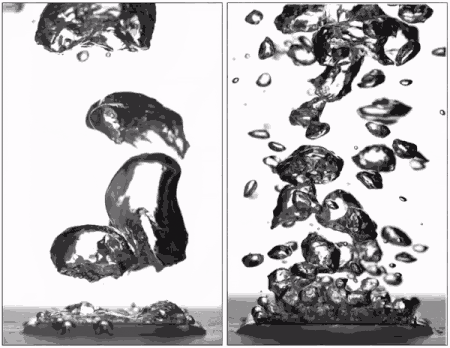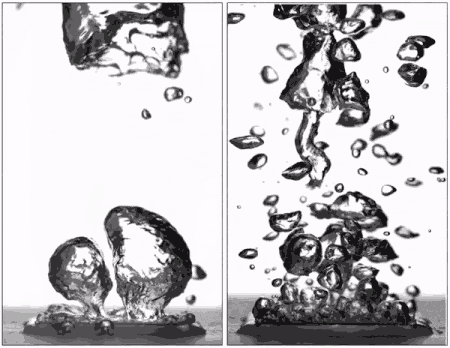
Superhydrophobic surfaces are normally insulated by vapor when brought to a boil (left), but getting water to stick makes them boil very efficiently (right), better than hydrophilic surfaces. (Purdue University image/Taylor Allred).
Superhydrophobic materials typically have a blanket of vapour on the surface, which is a very good heat insulator stopping any cooling. However, surfaces that repel water can support efficient boiling if all the air and vapour is removed. Without this layer of insulating vapour, superhydrophobic surfaces could potentially boil at lower temperatures than hydrophilic ones – delivering that greater cooling.
Greater cooling also promises greater performance. “Keeping the electronic device operating at the temperature it has to be at to prevent it from burning up and not being reliable is what limits the performance in many systems,” Weibel explained to Electropages.com. “It’s not that the device couldn’t do much more or be run at a higher power, it’s that there is no good means to take the heat out and that is the bottle neck [to better performance].”
One approach to remove the vapour is to create a texture on the hydrophobic surface to help it hold water. Previous attempts to achieve this have not created an entirely vapour free surface. Microstructured and nanostructured superhydrophobic surfaces were created by Purdue researchers. They then heated the surrounding water, being careful not to boil directly from the surface itself. This removes the layer of air that is normally trapped within the texture of the superhydrophobic surface. This is achieved because the small bubbles that occur during boiling are able to rise from the surface and do not coalesce into that vapour blanket.
The greater cooling is possible because hydrophobic materials enable the formation of many more small bubbles than hydrophilic surfaces. The Purdue research was part of the United States Navy’s Naval Enterprise Partnership Teaming with Universities for National Excellence (NEPTUNE) and was funded by the Office of Naval Research within NEPTUNE’s Center for Power and Energy Research.
Read More: Solar micro inverter market strengthens on PV demand
