Advanced Anode-Electrolyte Theory for Ammonium Ion Batteries
17-04-2024 | By Liam Critchley

Key Things to Know:
- Advancing AIB Technology: Ammonium Ion Batteries (AIBs) offer a safer, more environmentally friendly alternative to traditional batteries, using ammonium acetate (NH4Ac) which prevents dendritic growth and enhances battery life.
- Innovative Anode Materials: Recent developments in anode materials such as MXenes, explored through DFT calculations, show promising low voltage performance and high energy density.
- Pseudocapacitive Behavior: MXene anodes in AIBs exhibit unique pseudocapacitive behavior with NH4Ac, achieving high capacity and stability over 5000 cycles, a significant advancement over other materials.
- Research-backed Improvements: Studies like those from Sun et al. confirm the benefits of using ammonium acetate, enhancing the overall safety and effectiveness of AIBs.
Many battery architectures now exist as engineers and scientists try to pivot from Li-ion batteries to try and bring new battery systems that have high energy densities, can charge faster, or are more environmentally friendly. In recent years, many new battery architectures have been commercialised after years of academic development, and other new batteries are starting to take their place in the academic world.
One of these batteries are ammonium ion batteries (AIBs), which use aqueous electrolytes, so are safer and less prone to thermal runaway. AIBs have a low cost, inherent stability, good electrochemical properties, and are environmentally friendly. This is a newer area of battery research compared to some of the more established systems, and while there’s the potential for creating more environmentally friendly batteries, AIBs have been held back by challenges in anode design.
The Potential of Ammonium Batteries
AIBs are rechargeable batteries that use ammonium ions (NH4+) as the charge carriers. Compared to traditional metal ions— Li+, Na+, K+, Zn2+ and Mg2+—ammonium ions have fast diffusion characteristics in aqueous (water-based) electrolytes due to having a smaller ionic radius and lighter molar mass. Ammonium ions also don’t experience dendritic growth—which is often responsible for battery short-circuiting—unlike many metal-ion batteries, so there’s the potential to create safer batteries that degrade slower. Ammonium ion electrolytes are also more environmentally friendly than the organic solvents used in metal-ion batteries and are much cheaper as well. One of the most common electrolytes is ammonium acetate (NH4Ac).
To further underscore the environmental benefits and operational safety of AIBs, recent studies, such as those by Sun et al., highlight the role of ammonium acetate (NH4Ac) electrolytes in enhancing the stability and capacity of these batteries. Unlike traditional electrolytes, NH4Ac helps prevent the formation of dendrites, which are often a major safety hazard in battery operations. This characteristic not only improves safety but also significantly extends the battery's lifecycle, making AIBs a more sustainable option in the long term.
All these characteristics show that there’s a lot of potential for AIBs, especially as these characteristics are often seen as advantages over metal-ion batteries—which have become the commercial standard today. A lot of work has gone into the cathode design of AIBs, and high specific capacities can now be achieved. Cathode examples include nickel-based, manganese-based, and vanadium-based materials. However, not as much effort has gone into the anode design, and the interplay between the anode and the electrolyte is an area that still needs to be developed further.
Developments in New Anode Materials
The anode is currently the limiting factor for AIBs, as there are not many anode materials to date that have a low working voltage. The development of AIB anodes has been primarily limited to transition metal oxide/sulphides and organic polymers. These are not ideal materials because the transition metal compounds tend to have poor capacities, and the polymer anode has a high dissolution rate, which leads to poor cycling stability. This has rendered a lot of AIBs developed to date to be unfeasible for practical applications.
Despite the material challenges, new materials continue to be proffered and trialled. One class of materials that have been touted as a potential solution is MXenes. MXenes are 2D materials that are created from a MAX phase material, which has the general formula of Mn+1AXn. In this chemical structure, the M is an early transition metal, A is a group 13 or 14 element and X is either carbon or nitrogen.
MXene’s have a range of properties that are beneficial for electrodes. For example, they have excellent electrical conductivity and high surface activity, and the way that the layers stack on top of each other is desirable for ion storage—especially compared to other types of nanomaterials. So, MXenes are seen as a potential benefit for AIBs, and a lot of theoretical work is currently being done to see if they’re worth exploring further experimentally.
The potential of MXenes as effective anode materials in Ammonium Ion Batteries is supported by detailed Density Functional Theory (DFT) calculations. As illustrated in Figure 1 below, these calculations reveal the low working potential and high pseudocapacitive behavior of MXene materials, specifically V2CTx, which suggest their suitability for high-performance applications in battery technology.
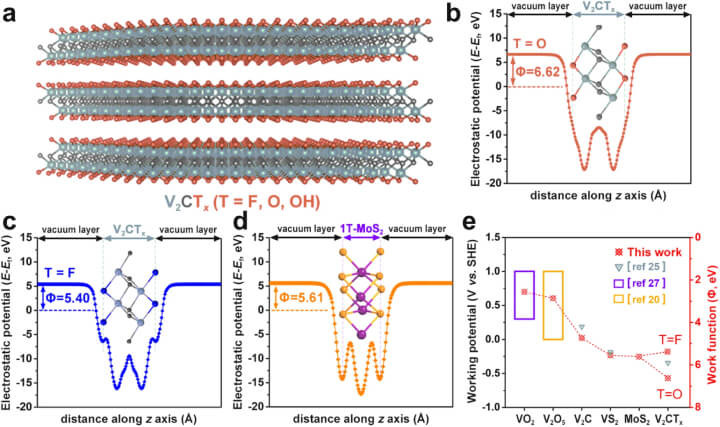
a The lateral perspective of the structural model for V2CTx MXene is depicted. The spheres colored in grey, black, and orange represent V, C, and T terminations, correspondingly. b, c The plane-averaged electrostatic potential curves for V2CO2 and V2CF2 are shown respectively. d The plane-averaged electrostatic potential curve for 1T-MoS2 is illustrated. e The correlation between the work function and the operational potential window of the two-dimensional materials studied in this research is demonstrated.
In corroborating the suitability of MXenes for AIB applications, research outlined in Nature Communications by Sun et al. discusses the pseudocapacitive properties of V2CTx MXenes when used with NH4Ac electrolytes. These materials not only promise lower working potentials but also demonstrate enhanced stability and energy density, crucial for high-performance batteries. The inherent high surface activity of MXenes enhances their efficiency, significantly advancing anode fabrication in sustainable battery technologies.
DFT Calculations Suggest Best Aqueous Electrolyte for MXene Anodes
Density functional theory (DFT) is a well-established technique in the field of theoretical and computational chemistry and is often used to model and probe the properties of different material systems to see if they are feasible for a particular application (as well as to deduce their structure). DFT calculations have shown that the MXene, V2CTx, has the lowest working potential window compared to other vanadium-based materials, so it has the potential to be a promising anode material for AIBs.
Researchers have now looked further into this MXene anode using a combination of DFT calculations and physical material characterisation methods to see how it interplays with the aqueous electrolyte and to investigate what the performance of these anodes might look like. The researchers found that this MXene anode exhibits a pseudocapacitive behaviour for ammonium ion storage, with a specific capacity of 115.9 mAh g-1 at 1 A g-1 and a capacity retention of 100% after 5000 cycles at 5 A g-1.
Despite many aqueous electrolytes being trialled with the MXene anode—including (NH4)2SO4, NH4Cl, (NH4)2C2O4, and NH4Me—the only electrolyte where the MXene anode showed this pseudocapacitive behaviour was with the NH4Ac ammonium acetate electrolyte. The pseudocapacitive behaviour performance exhibited between the MXene anode and the NH4Ac electrolyte surpasses all capacitive-typed electrodes in AIBs to date.
To probe the properties of the anode and its interaction with the electrolyte, in-situ electrochemical quartz crystal microbalance (EQCM) measurements were taken which showed a two-step electrochemical process that generates the pseudocapacitive storage behaviour in the ammonium acetate electrolyte.
The experimental results were followed up by DFT calculations and simulations to better understand this electrochemical process. The first part of the process involved the electrostatic adsorption/deposition of NH4+ on the MXene surface. In the second step of the process, a redox reaction takes place between the d-V2CTx groups of the anode and the [NH4+(HAc)3] groups of the electrolyte. During this redox process, the central NH4+ ion acts as a pseudo-proton, which facilitates the alternation of V2CTx terminations. This then changes the valence state of the vanadium ions in the complex, promoting charge transfer.
The innovative use of ammonium acetate in MXene-based AIBs not only improves the electrochemical stability but also enhances the energy storage capacity as detailed by Sun et al. This breakthrough is attributed to the unique interaction between the NH4+ ions and the electrolyte, which facilitates efficient ion transport and robust cycle stability, laying the groundwork for next-generation energy storage solutions.
The researchers also confirmed the acetate ion enhancement effect in a MoS2-based (another 2D material in the transition metal dichalcogenide family) ammonium-ion battery. The ability to be used with different materials means that the acetate electrolyte could be used alongside different 2D materials to improve the capacity of AIBs. This approach also has the potential to break the capacity limitations in both Faradaic (redox) and non-Faradaic (electrostatic interactions only) and could offer a way to improve different AIBs and lead to the creation of more sustainable batteries.
Environmental Impact and Safety Considerations of Ammonium Ion Batteries
The shift towards Ammonium Ion Batteries (AIBs) is not only a technical improvement over traditional battery systems but also a significant step forward in environmental conservation. AIBs utilise less hazardous materials and involve safer chemical reactions, which reduces the risk of accidents and decreases environmental pollution. Studies like those from Sun et al. in Nature Communications have shown that the use of safer electrolytes like ammonium acetate significantly mitigates risks such as thermal runaway and chemical leaching, making AIBs a preferred choice for sustainable energy storage.
Reference:

